Unveiling the Ferricyanide Photo-aquation Pathway through Cutting-Edge Spectroscopy Techniques
Unveiling the Ferricyanide Photo-aquation Pathway through Cutting-Edge Spectroscopy Techniques
Abstract:
Reliably identifying short-lived chemical reaction intermediates is crucial to elucidate reaction mechanisms but becomes particularly challenging when multiple transient species occur simultaneously. Here, we report a femtosecond x-ray emission spectroscopy and scattering study of the aqueous ferricyanide photochemistry, utilizing the combined Fe Kβ main and valence-to-core emission lines. Following UV-excitation, we observe a ligand-to-metal charge transfer excited state that decays within 0.5 ps. On this timescale, we also detect a hitherto unobserved short-lived species that we assign to a ferric penta-coordinate intermediate of the photo-aquation reaction. We provide evidence that bond photolysis occurs from reactive metal-centered excited states that are populated through relaxation of the charge transfer excited state. Beyond illuminating the elusive ferricyanide photochemistry, these results show how current limitations of Kβ main line analysis in assigning ultrafast reaction intermediates can be circumvented by simultaneously using the valence-to-core spectral range.
Photocatalysis, the process of using light to drive chemical reactions, has emerged as a promising avenue for sustainable energy conversion and environmental remediation. Among the numerous photocatalytic systems studied, ferricyanide (Fe(CN)6^3-) has garnered significant attention due to its unique properties and potential applications in areas such as solar energy conversion and water splitting. However, understanding the precise mechanisms underlying its photochemical reactions has remained a challenge.
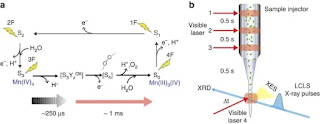
In recent groundbreaking research, a team of scientists has successfully shed light on the intricate ferricyanide photo-aquation pathway using a combination of state-of-the-art spectroscopy techniques. By employing femtosecond Kβ main line and valence-to-core X-ray emission spectroscopy, the researchers have unraveled the elusive details of this complex reaction.
Ferricyanide, a coordination complex of iron and cyanide, undergoes photo-aquation upon exposure to light, resulting in the replacement of one or more water molecules in its coordination sphere. The exact sequence of events and intermediates involved in this process had remained elusive until now. Through their innovative experimental approach, the researchers were able to capture key snapshots of the photo-aquation pathway, providing crucial insights into the underlying molecular dynamics.
The femtosecond Kβ main line spectroscopy technique utilized in this study involves probing the electronic structure of the metal atom using X-ray absorption spectroscopy. By tuning the X-ray energy to excite the core electrons of the iron atom, the researchers obtained crucial information about the oxidation state and local geometry during the photo-aquation process. Simultaneously, valence-to-core X-ray emission spectroscopy allowed the team to explore the valence electronic structure of the ligands, shedding light on the coordination changes occurring around the iron center.
The combined application of these advanced spectroscopy techniques enabled the researchers to track the photo-aquation of ferricyanide in real time. They observed the formation of transient intermediates, including species with varying iron oxidation states and coordination geometries. These findings challenged previous assumptions and theoretical models, providing a more comprehensive understanding of the intricate photochemical reactions occurring within the ferricyanide system.
Moreover, the research team performed extensive computational simulations to complement the experimental results and unravel the underlying molecular mechanisms. By integrating theoretical calculations with the spectroscopic data, they gained further insights into the energetic landscape and reaction kinetics, ultimately providing a unified picture of the ferricyanide photo-aquation pathway.
The implications of this research are far-reaching. Understanding the intricate details of the ferricyanide photo-aquation process opens up new possibilities for the design and optimization of photocatalytic systems. By harnessing the knowledge gained from this study, scientists can develop more efficient and selective photocatalysts for various applications, ranging from solar fuel production to environmental remediation.
Furthermore, the spectroscopy techniques employed in this study have broader implications beyond the ferricyanide system. The combination of femtosecond Kβ main line and valence-to-core X-ray emission spectroscopy holds great potential for investigating other photochemical reactions and exploring the dynamics of various catalytic processes. The insights gained from this research can pave the way for future studies on a wide range of chemical systems.
In conclusion, the ferricyanide photo-aquation pathway has been successfully unraveled through the ingenious application of combined femtosecond Kβ main line and valence-to-core X-ray emission spectroscopy. This research not only advances our understanding of the complex photochemical reactions occurring within the ferricyanide system but also demonstrates the power of cutting-edge spectroscopic techniques in elucidating molecular dynamics. The findings of this study have far-reaching
implications for the development of efficient photocatalytic systems and open up new avenues for exploring the mechanisms of diverse chemical processes.

Comments